Emerson Stokes Research Group @ Mississippi State University
Welcome to the Emerson Stokes Research Group at Mississippi State University. We are a diverse group of scientists interested in studying novel reactions and processes at the interface of chemistry, biology, and materials. Most of the chemical and biochemical systems under investigation in the ESRG are focused on the chemistry of first-row transition metal ions, where their ligand archtecture plays a key role in controlling their chemistry. To study these systems we use tools from biology, biochemistry, biophysics, organic chemistry, inorganic chemistry, and catalysis. If you are interested in collaborating with us or joining our team, please feel free to contact us!
News

Researchers wanted!
Emerson Stoke Research Group is recruiting undergraduate and graduate student researchers to join our growing reserach team. Students with backgrounds in molecular biology, biochemistry, microbiology, chemistry, chemical engineering, biomedical engineering, and others relavent fields should take a look at our research page. If you see something interesting, please contact Dr. Sean Stokes or Dr. Joe Emerson to get involved.
_________________________________________________________________
_________________________________________________________________

ESRG welcomes new researchers!
The lab has dramatically expanded during the 2021-2022 academic year, where 4.5 new graduate students have gotten involved in ESRG projects. This trend appears to be continuing this summer, where we welcome Katie Power as a prospective new graduate student. We also have have included 3 new (full-time) undergraduate researchers to the group. MSU students Erin Matthews and Alison Duckworrth started summer projects in May 2022. Finally NSF REU student Emily Rouse (Lebanon Valley College) has joined the group in June. This latest addition of researchers has allowed us to shift to a higher gear!
Welcome Emily, Erin, Alison, and Katie!
_________________________________________________________________
Welcome Emily, Erin, Alison, and Katie!
_________________________________________________________________
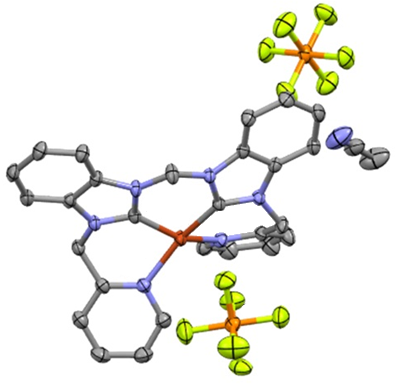
ESRG Post-doctoral fellows
Mitu Sharma and Selvam Raju have joined the Emerson Stoke Research Group in September 2021. This addition of well trained scientists in inorganic chemistry and organic synthesis will allow the ESRG to turn some new corners!
Welcome Drs. Sharma and Raju!
Welcome Drs. Sharma and Raju!
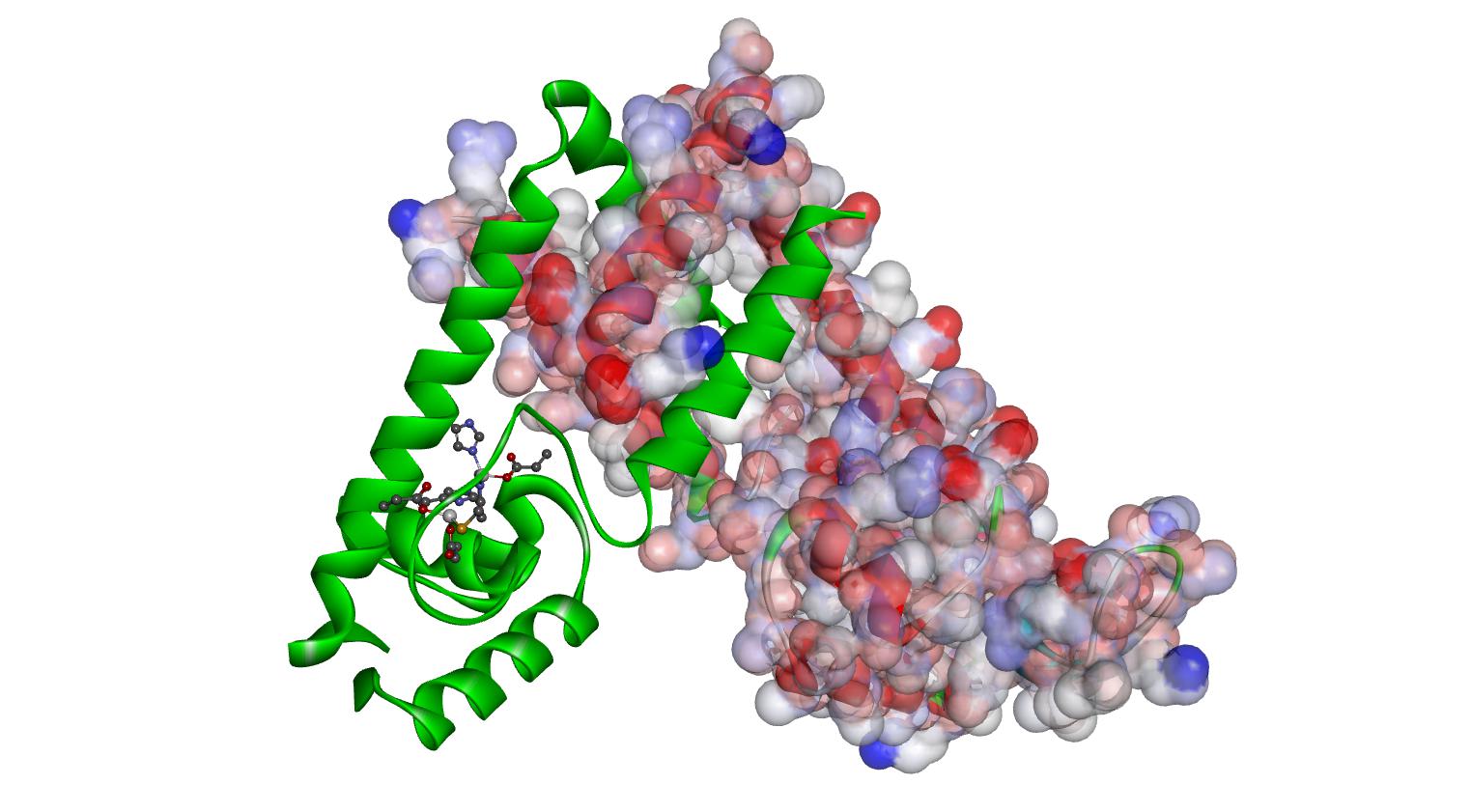
Emerson and Stokes are selected to join the NIH/MSU COBRE program!
The research collaboration Emerson and Stokes has been selected to join the National Institute of Health’s Center of Biomedical Research Excellence in Pathogen Host Interactions Program at Mississippi State University. Their project entitled "Conformational dynamics of the zinc(II) binding site of AdcR and its role in DNA binding" will receive $830K over the next 2.5 years to support this work.

